To place orders, set up an account, or become a USA reseller, call 877-722-8910
Fastep® COVID-19 Antigen Home Test (FDA Emergency Use Authorization Granted)


| Bulk orders for: | |
|
|
| call 877-722-8910 ext 108 | |
Carolina Liquid Chemistries is now offering the FDA Emergency Use Authorized Fastep® COVID-19 Antigen Home Test
Product Description
The Fastep® COVID-19 Antigen Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid antigen from SARS-CoV-2.
This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older or adult collected anterior nasal (nares) swab samples from individuals aged two years or older. This test is authorized for individuals with symptoms of COVID-19 within the first 5 days of symptom onset when tested twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested at least three times over five days with at least 48 hours between tests.
The Fastep COVID-19 Antigen Home Test does not differentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen, which is generally detectable in anterior nasal (nares) swab specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Individuals who test positive with the Fastep COVID-19 Antigen Home Test should self-isolate and seek follow up care with their physician or healthcare provider as additional testing may be necessary.
All negative results are presumptive and confirmation with a molecular assay, if necessary for patient management, may be performed. Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control measures such as isolating from others and wearing masks. Negative results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19.
Individuals who test negative and continue to experience COVID-19 like symptoms of fever, cough and/or shortness of breath may still have SARS-CoV-2 infection and should seek follow up care from their physician or healthcare provider.
The Fastep COVID-19 Antigen Home Test is intended for non-prescription self-use and/or as applicable an adult lay user testing another person 2 years of age or older in a non-laboratory setting. The Fastep COVID-19 Antigen Home Test is only for use under the Food and Drug Administration’s Emergency Use Authorization. This product has not been FDA cleared or approved.
Individuals should provide all results obtained with this product to their healthcare provider for public health reporting and to receive appropriate medical care. All healthcare providers will report all test results they receive from individuals who use the authorized product to relevant public health authorities in accordance with local, state, and federal requirements using appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests provided by the CDC.


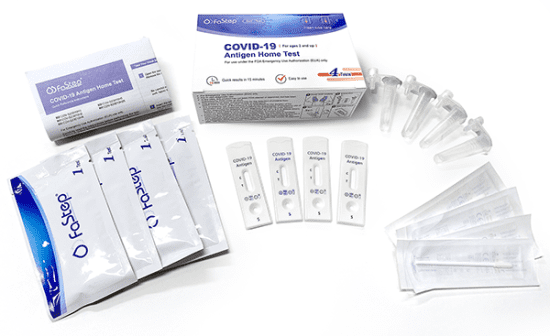
Above: 2-pack contents. Below: 4-pack contents.

Other COVID-19 Related Products
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Important Information
The Fastep® COVID-19 Antigen Home Test has not been FDA cleared or approved; this test has been authorized by FDA under an Emergency Use Authorization (EUA). This test has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 is terminated under Section 564(b)(2) of the Act or the EUA is revoked under Section 564(g) of the Act.

